A Brief Tour of Coffee’s Chemical Composition
Every day millions of people around the world begin their day religiously with a morning cup of coffee. Though today we easily identify coffee in its beverage form, it wasn’t always this way in the beginning. Throughout history, coffee has taken on several physical transformations, initially serving as an energy source when nomadic tribes combined coffee berries with animal fat as an early form of an energy bar.
Later it was consumed as tea, then wine, and finally as the beverage, we’ve come to identify today. Since the beginning, coffee has always been a product of great mystery, having been discovered accidentally in wild forests of Abyssinia (Ethiopia) and consumed in its native cherry form, then later, passed through fire to significantly alter its chemical state.
And although coffee has been in existence for thousands of years, it’s only been in the past half-century or so, that scientists have been able to truly identify and understand what exactly is contained in this mystical bean. To date, scientists have identified over 1,000 compounds in coffee, which when compared to products such as wine or chocolate that are composed of a few hundred, pale in comparison to that of coffee.
Luckily through advancements in technology, much of coffee’s chemical makeup has been unlocked and we now have a better perspective on the chemistry contained within this mystical bean.
Caffeine
For many, coffee drinking is simply a delivery medium for a potent alkaloid we have come to identify as caffeine or technically as 1,3,7 – trimethylxanthine. Although caffeine is strongly associated with coffee, its production within the plant kingdom is not exclusive but is seen throughout several other forms of plant life.
Mate, for example, which is traditionally consumed in parts of Uruguay and Argentina, contains less than one percent by weight. Whereas, tea leaves (Camellia Sinensis) which originated in China, contain almost three times the concentration of caffeine than Arabica, with Brazilian mate almost twice that of robusta coffee.
Turns out that Mother Nature was quite generous when it came to distributing caffeine amongst the plant kingdom. But for humans, caffeine is very unique. Thus far we are the only living forms on Earth that readily seek caffeine for both its stimulatory and psychological effects.
For all other life forms, caffeine is a potent toxin capable of sterilization, phytotoxicity, and antifungal properties. As such scientists believe that caffeine, with its intensely bitter taste, has evolved as a primitive defense mechanism in coffee ensuring its survival in the wild for thousands of years. It’s no surprise then, that the caffeine content of the more “robust” Robusta species is almost double that of the more delicate Arabica.
The belief is that as insects attack the coffee cherry, they are deterred by the bitter taste of caffeine and simply move on to the next crop. Since Arabica is typically grown at higher altitudes than Robusta, where the attack of insects is reduced, Arabica has evolved to produce less caffeine.
Lipids
Lipid production and its subsequent survival after the roasting process play an important role in overall coffee quality. In general, most of the lipids exist in the form of coffee oil and are located within the endosperm (bean) of the cherry, with only a small percentage deposited onto the outer portion of the coffee wax. Coincidentally, scientists have analyzed and discovered that much of the chemical makeup of coffee oil is very similar to that of vegetable cooking oils.
As such, much of the lipid content in coffee remains unchanged and relatively stable even at the elevated temperatures associated with roasting. In its green form, both Arabica and Robusta coffee contain on average 15-17% and 10-11.5%, respectively. But because Arabica contains about 60% more lipids than Robusta, many believe this stark difference is one reason responsible for the quality difference between both species.
Thus far, the claim has remained unconfirmed, until French scientists recently discovered a direct correlation between lipid content and overall cup quality. It turns out that as lipid content increases within the bean, so does overall cup quality. It’s a very plausible explanation when one considers that the majority of important flavor compounds in coffee are also fat soluble.
Carbohydrates
Carbohydrates make up roughly fifty percent of coffee’s total dry weight by composition. After roasting, remaining carbohydrates in the cup contribute to mouth-feel or body, with some studies suggesting they are also responsible for the quality of the foam common in espresso beverages. Although there are numerous types of carbohydrates in coffee, perhaps the most important is that sucrose. Sucrose, or more commonly known as table sugar, makes up 6-9% of Arabica with a slightly less (3-7%) amount contained in Robusta coffee.
During roasting, sucrose is readily decomposed and studies have shown that up to 97% of the initial sucrose content is lost even at light roasts. Its role during roasting is enormous with a large portion of the available carbohydrates participating in the Maillard and numerous other secondary reactions. One class of important byproducts created during roasting is organic acids. In its native green form, coffee contains negligible amounts of formic, acetic, and lactic acid.
Though once roasted, there is an exponential increase in aliphatic acid production, along with a paralleled increase in coffee acidity.
Since acidity plays an important role in assessing quality, it’s no surprise why see typically see higher levels of perceived acidity in Arabica coffee than Robusta, due in part, to its higher sucrose concentration. Coincidentally, in the past year, Brazilian scientists have identified a single gene, sucrose synthase, which controls sucrose production in plants and may hold the key to cultivating higher-quality coffee for years to come.
Proteins
Protein content for both green Arabica and Robusta coffee varies between 10-13% and exists as free or bound proteins within the coffee matrix. Although actual concentrations can vary, several factors can affect free protein content, including improper storage which may increase free protein levels and lead to detrimental effects on quality.
During roasting, proteins combine with carbohydrates in what is perhaps the most important reaction for all thermally processed foods – the Maillard Reaction. This set of reactions, discovered by a French chemist in 1910, is what is largely responsible for transforming the mere handful of compounds found in green coffee into the complex matrix that coffee is today.
As temperatures reach 150C (302F), the Maillard reaction propels free proteins in coffee to combine with reducing sugars, ultimately leading to the formation of hundreds of important aromatic compounds. Of these, pyrazines and pyridines have the greatest aromatic contribution and are responsible for the distinct maize/nutty aromas found in coffee. The reaction also leads to the formation of brown-colored polymetric melanoidins – the compounds responsible for coffee’s color.
Coincidentally, this is the same set of reactions that gives rise to the alluring aromas we generate when toasting a loaf of bread or grilling a piece of steak. Although many of the byproducts created during the Maillard reaction are beneficial to coffee, in other agricultural products, these set of browning reactions can be a serious detriment to quality.
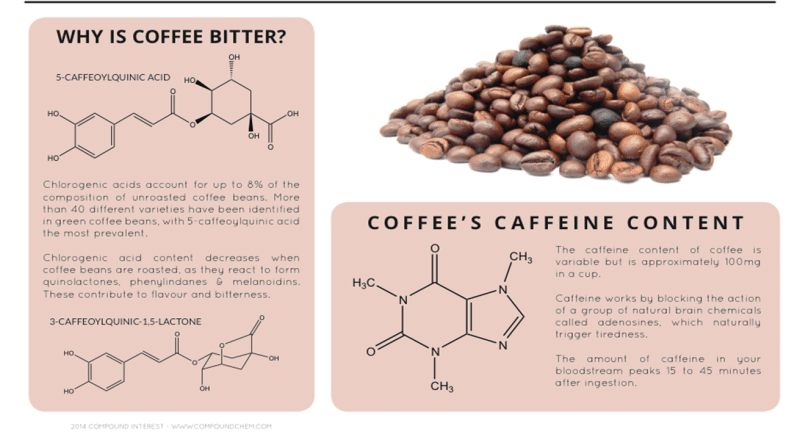
In the cup, proteins also play a role in taste by forming secondary compounds during the roasting process. It turns out that the majority of coffee’s “bitterness” is not due solely to caffeine, but rather bitter compounds produced during the Maillard reaction. Caffeine, as intensely bitter as it is, accounts for only 10-20% of coffee’s total bitterness.